No More Mistakes with Flour Mill Machine Manufacturer
Mar 11 2023
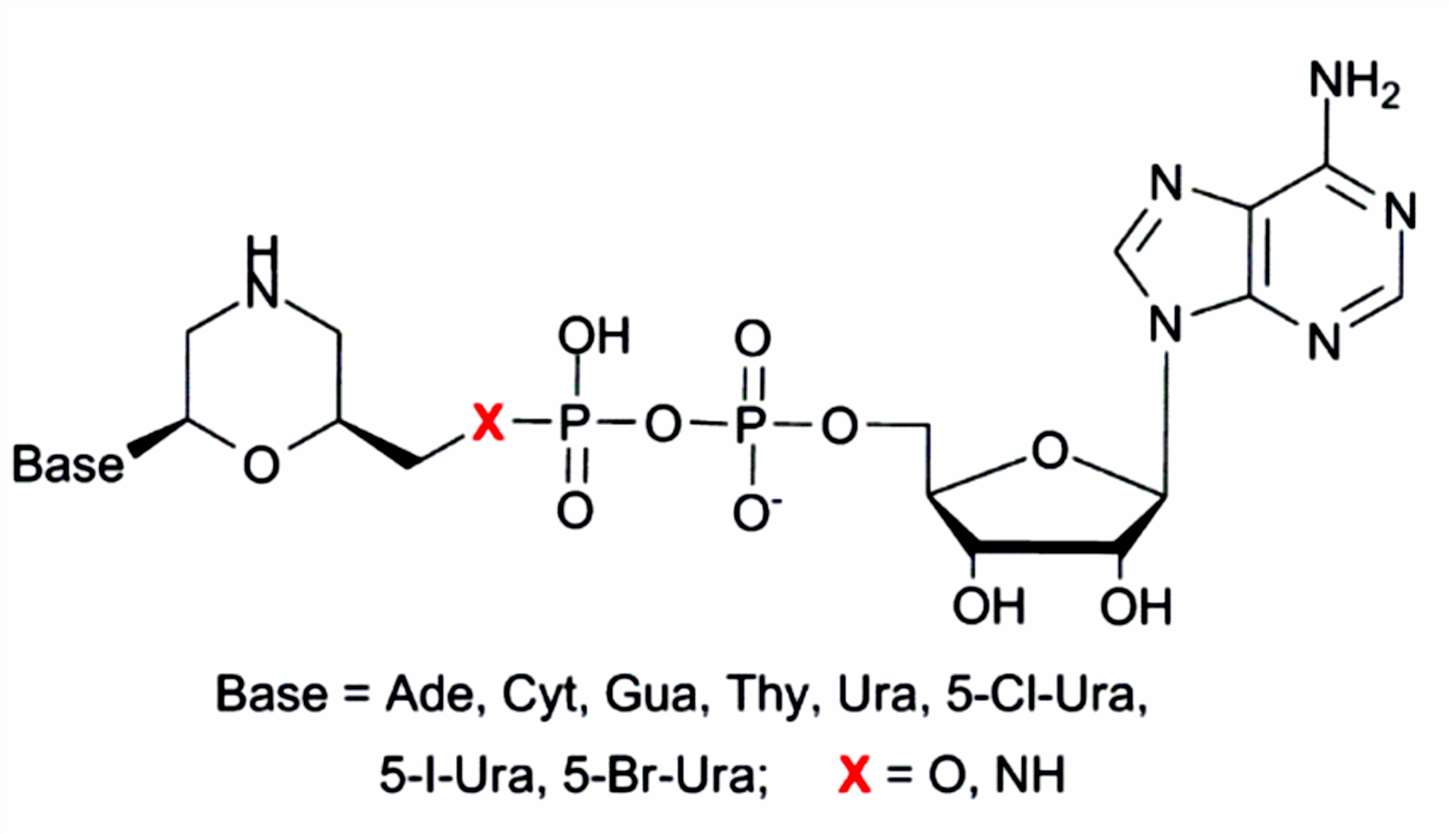

Morpholine nucleoside analogs are synthetic nucleoside analogs in which the sugar-phosphate backbone of the natural nucleotide is replaced by a morpholine ring linked by a phosphorodiamine bond. This structural transformation fundamentally alters the physicochemical and biological properties of the molecule. Unlike the negatively charged ribose-phosphate backbone of DNA and RNA, morpholine nucleoside analogs present an electrically neutral backbone at physiological pH, thereby enhancing their biocompatibility and resistance to degradation.
Morpholine nucleoside analogs regulate gene expression primarily through an antisense mechanism. By binding to complementary RNA sequences, they physically block ribosome or spliceosome advancement, thereby preventing translation initiation, elongation, or splice site recognition.
Unlike siRNAs or shRNAs that depend on cellular RNA interference (RNAi) pathways and RNase H recruitment, morpholino acts by directly occupying the target mRNA region. This action is particularly applicable to the regulation of variable splicing events, exon skipping, or translation initiation site repression. For example, in the treatment of Duchenne muscular dystrophy (DMD), morpholine oligonucleotides have been used to induce exon skipping, thereby restoring the open reading frame of the dystrophin gene. This spatial blocking mechanism offers advantages in terms of predictability, specificity and reduced immune activation.
Alfa Chemistry offers a wide range of morpholino-based intermediates, including monomers designed for therapeutic oligonucleotide assembly. These materials have driven innovation in gene therapy, particularly in the treatment of genetic diseases such as Duchenne muscular dystrophy, for which phosphorodiamidate morpholino oligomer (PMO)-based drugs such as eteplirsen have been approved.
The synthesis of morpholino nucleoside analogues involves the strategic incorporation of nucleobases onto a morpholine scaffold with defined stereochemistry. A recent strategy emphasizes the preparation of optically pure 6-hydroxymethyl-morpholine acetals as versatile intermediates. The synthetic route begins with the oxirane ring opening of optically pure (S)-glycidol using N-nosyl aminoacetaldehyde as the nucleophile. This reaction yields a chiral intermediate that undergoes tandem O-benzoylation and ring-closure to form the morpholine ring, preserving stereochemical fidelity essential for biological activity.
The condensation of this intermediate with canonical nucleobases - such as thymine or adenine - under Lewis acid catalysis (e.g., trimethylsilyl triflate or SnCl4) enables selective glycosylation at the anomeric center. Notably, the choice of catalyst dictates the stereoselectivity of the final product: TMSOTf favors biologically preferred β-anomers, while SnCl4 predominantly yields previously unsynthesized α-anomers. This controllable anomeric outcome expands the chemical diversity of morpholino nucleoside analogues and facilitates structure-activity relationship (SAR) studies.
Morpholine nucleoside analogs have demonstrated promising therapeutic potential in several disease areas, particularly in the areas of genetic disorders, viral infections and cancer.
Morpholine nucleoside analogs play a central role in antisense therapy, which works by blocking the translation of defective mRNAs associated with genetic diseases. For example, PMO has been successfully used to treat DMD by inducing exon skipping to restore functional dystrophin proteins. Unlike conventional antisense oligonucleotides, PMO is uncharged, which minimizes interaction with serum proteins and reduces toxicity.
In antiviral therapy, morpholino oligomers have been designed to target viral RNA sequences with great precision. Experimental models have demonstrated that these analogs are effective in inhibiting the replication of West Nile virus, Ebola virus and influenza virus. Notably, some designs have also shown potential in preclinical models targeting the SARS-CoV-2 genome, suggesting a broad antiviral spectrum.
In oncology, morpholino-substituted nucleoside analogs can be used as tools to down-regulate oncogenic gene expression or alter tumor-associated mRNA splicing. By blocking the translation of proteins essential for cancer cell proliferation or survival, these analogs can induce cell growth inhibition or apoptosis. Morpholino-substituted oligonucleotides are also being explored in combination with CRISPR-Cas9 and other genome editing technologies as modulators of gene editing fidelity and efficiency.
In addition, these analogs are valuable in functional genomics and drug screening platforms. They can identify key gene functions, validate therapeutic targets and accelerate the development of lead compounds.
The stereochemical conformation of the C-6 position of the morpholine ring is critical for the recognition and binding of these analogs to target RNA sequences. The use of optically pure glycidol during synthesis ensures consistent stereochemical configurations, thereby facilitating reproducible biological reactions. For example, the β-terminal isomeric morpholine monomer exhibits higher hybridization affinity and biocompatibility compared to the α-terminal isomer.
This stereochemical precision also contributes to the overall efficacy of oligonucleotide drug products, as mismatched or racemic mixtures may decrease target specificity or increase immunogenicity. Thus, stereoselective synthetic control achieved through the described Lewis acid-promoted glycosylation strategy is critical in therapeutic development.
Research continues to expand the chemical space of morpholine nucleoside analogs. Ongoing studies include exploring different nucleobase functions, enhanced water solubility, and improved in vivo delivery through nanoparticle encapsulation or conjugation with targeted ligands. In addition, expanding the range of nucleophilic reagents that react with morpholine intermediates may yield novel analogs with unique mechanisms of action.
Synthesis methods such as those described above - which enable a high degree of end-isomer control, modular assembly and scalability - are essential to accelerate the drug development process. Alfa Chemistry has deep expertise in nucleoside chemistry, providing basic research materials and custom synthesis services to facilitate innovation in oligonucleotide therapeutics.
Social Media Marketing Strategies for Beginners
Mar 14 2023
(0) Comments